www.industryemea.com
27
'22
Written on Modified on
Foba: From data base to direct marking, Digital solution for an efficient and validation-proof UDI process
The challenges for medical technology companies in connection with UDI are still on the rise. Companies therefore need to make strategic decisions shortly to optimize their UDI process, especially regarding its digitization. After all, the next MDR implementation deadline is approaching: UDI direct part marking becomes mandatory for class III products as of May 2023.

Until 2027, more MDR-implementation deadlines for the European market will follow. Meanwhile, with the final FDA-deadline on September 24, 2022, the UDI implementation in the U.S. market is completed. The MDR specifications are similar to those of the FDA, but require specific approaches, e.g. for EUDAMED registration, involvement of notified bodies in charge or for direct marking application. Even though the European database EUDAMED is delayed in becoming operational, the deadlines for UDI labeling are not changing.
The UDI system is complex and affects the entire medical device manufacturing process, from product design to placing on the market. Therefore, it often causes difficulties to align responsibilities within the company and to precisely coordinate the cooperation of those responsible, for example between process management, quality management, data processing, project management, and production.
At the end of this process, laser marking is a decisive step that ensures the traceability of each individual product and thus patient safety in medical practice.
The prerequisite for this: consistent error-free data processing and, finally, correct product marking. The marked UDI code must be reliably and permanently readable.

How can the effort associated with UDI requirements be reduced?
To make workflows more efficient, standardize processes and avoid extra work, digitization based on ERP (Enterprise Resource Planning) software can bring many benefits. After all, interlocking data processing can dovetail all steps in the company, from master data to serial direct marking. This also ensures optimal connection of production technology to ERP systems and databases.
Digitization measures in this area can be introduced at any time; both products already on the market and additional UDI data can be newly integrated into the system. Regarding the upcoming MDR, all UDIs that will be required for EUDAMED in the future can already be maintained in advance in the ERP.
The processing of this data is also the basis for the preparation of serial labeling for laser marking or the provision of the 2D UDI codes assigned per part.
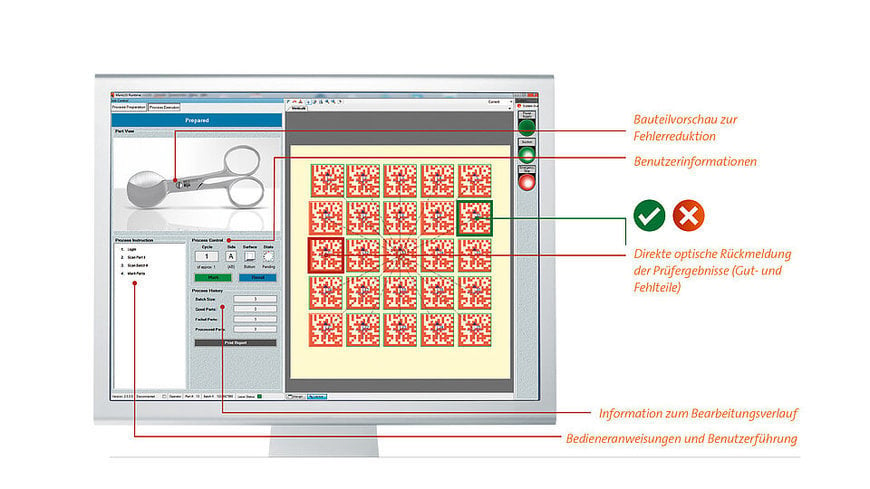
Medical device validation also includes data integrity
Medical device manufacturers should ensure that the ERP used is suitable for UDI labeling and that the underlying regulations (e.g. DIN EN ISO 13485, MDR, 21CFR820) are taken into account during digitization.
Preconfigured and validation-capable software solutions then facilitate not only the implementation, but also the validation required in medical technology. This is because the integrity of all relevant data is also part of the validation, which in turn is a mandatory for product quality and thus for patient safety.
www.fobalaser.com

